NASH orci interdum erat mollis
Etiam condimentum, arcu eget consectetur scelerisque, est mauris aliquet arcu, in condimentum purus dui ut tortor. Curabitur a neque sit amet enim posuere volutpat. Donec eget metus non dolor scelerisque placerat. Sed posuere neque at augue auctor, et pretium purus luctus. Cras quis lacinia mauris. Nam feugiat mi ut quam laoreet, mattis mattis felis hendrerit. Morbi in gravida ipsum. Aenean dictum odio orci, ut aliquet tellus eleifend quis. Vivamus dignissim metus eu molestie mattis.
Overview:
Etiam condimentum, arcu eget consectetur scelerisque, est mauris aliquet arcu, in condimentum purus dui ut tortor. Curabitur a neque sit amet enim posuere volutpat. Donec eget metus non dolor scelerisque placerat. Sed posuere neque at augue auctor, et pretium purus luctus. Cras quis lacinia mauris. Nam feugiat mi ut quam laoreet, mattis mattis felis hendrerit. Morbi in gravida ipsum. Aenean dictum odio orci, ut aliquet tellus eleifend quis. Vivamus dignissim metus eu molestie mattis.
Eligibility:
Etiam condimentum, arcu eget consectetur scelerisque, est mauris aliquet arcu, in condimentum purus dui ut tortor. Curabitur a neque sit amet enim posuere volutpat. Donec eget metus non dolor scelerisque placerat. Sed posuere neque at augue auctor, et pretium purus luctus. Cras quis lacinia mauris. Nam feugiat mi ut quam laoreet, mattis mattis felis hendrerit. Morbi in gravida ipsum. Aenean dictum odio orci, ut aliquet tellus eleifend quis. Vivamus dignissim metus eu molestie mattis.
Study Details:
Etiam condimentum, arcu eget consectetur scelerisque, est mauris aliquet arcu, in condimentum purus dui ut tortor. Curabitur a neque sit amet enim posuere volutpat. Donec eget metus non dolor scelerisque placerat. Sed posuere neque at augue auctor, et pretium purus luctus. Cras quis lacinia mauris. Nam feugiat mi ut quam laoreet, mattis mattis felis hendrerit. Morbi in gravida ipsum. Aenean dictum odio orci, ut aliquet tellus eleifend quis. Vivamus dignissim metus eu molestie mattis.
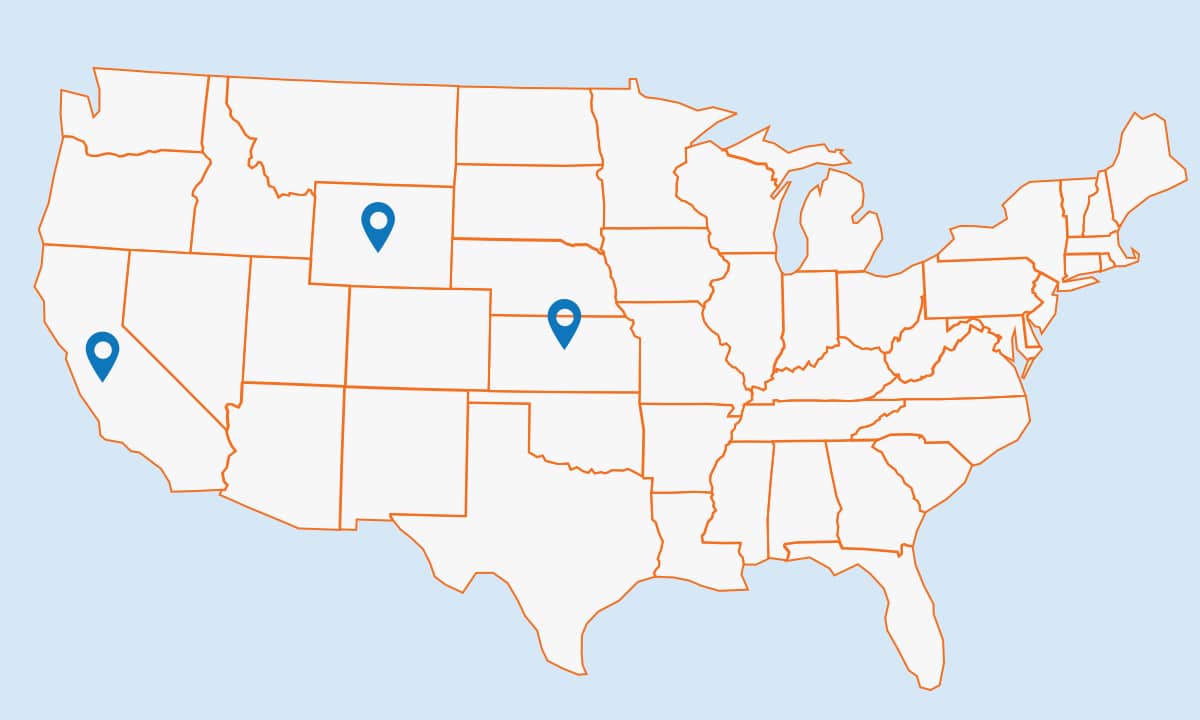
Study Locations
Questions? Reach out to learn more about the HARMONY Study:
Email: [email protected]
Phone: 650-765-8900

Pipeline
